Is Pb No3 2 Soluble or Insoluble in Water
Predict whether the compounds are soluble or insoluble in water. Lead II nitrates molar mass is 3312 grams per mole and its density is 453 grams per cubic centimeter.

Is Pb No3 2 Soluble Or Insoluble In Water Youtube
See the answer See the answer done loading.

. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent. Salts that are insoluble in water are made by precipitation. 2 is insoluble will be a solid because Pb 2 makes it insoluble Example 2.
K 2O is soluble because K is an alkali metal and is always soluble but MgO is insoluble because O 2-is insoluble unless bonded to. All Lead Pb compounds are water insoluble except PbNO32. All binary compounds of the halogens other than F with metals are soluble except those of Ag Hg I and Pb It is Hg-HgCl2 that is extremely insoluble ie Hg I that contains the weird Hg-Hg2 ion not HgCl2 that is reasonably soluble.
For the following equation provide the molecular equation. It commonly occurs as a colourless crystal or white powder and unlike most other leadII salts is soluble in water. All sodium salts are soluble in water.
Soluble - soluble more than 1g per 100g of water low - low solubility 001g to 1g per 100g of water insoluble - insoluble less than 001g per 100g of water not exist - do not exist in the aqueous environment. All Nitrates NO3- are water soluble. Is Pb NO3 2 soluble in water and why.
Indicate whether each of the following salts is soluble or insoluble in water and give a reason 1. Pb NO32 is the formula of lead II nitrate an inorganic compound whose water solubility is 52 grams per 100 milliliters at 20 degrees Celsius and 127 grams per 100 milliliters at 100 degrees Celsius. Is PbNO32 Lead II nitrate soluble or insoluble in water.
LeadII nitrate is an inorganic compound with the chemical formula PbNO32. All Sulphates are water soluble except Pb and Gp-II Ca to Ba compounds. Is HgNO32 soluble in water.
Lead II iodide PbI2 is insoluble in water. Pb NO32 Lead dinitrate is Soluble in water. Pb NO32 is soluble in water.
All nitrates are soluable in water so yes. H2SO4 Ba OH2 equation. We can also identify whether PbSO4 is soluble or insoluble in water according to the solubility rules which states most of the sulfates are soluble in water with exception of Ba 2 Ca 2 Pb 2 Ag 2 and Sr 2.
Reaction between zinc and lead 2 nitrate. Yes mercury II nitrate is soluble in water. Insoluble contains elements on the same line aka soluble insoluble elements 2.
The answer that Lead II hydroxide is insoluble in water. Although it is an ionic compound. For the following equation identify which reactants and products are solubleinsoluble in water.
View the full answer. Soluble Insoluble NH_Br CoBr Pb NO32 PbCI. Is PbOH2 Lead II hydroxide soluble or insoluble in water.
Yes its soluble in water. All Carbonates are water insoluble except Gp-I and Ammonium compounds. It is an ionic compound which readily dissociates i.
What is Soluble and Insoluble. The answer is that it is soluble in water. All Halides Cl- Br- I- are water soluble except Pb and Ag.
All nitrates are soluble in water. 3765 gL 0 C. KI Pb NO32 equation.
SrNO 32 is soluble because NO 3-is always soluble. Soluble elements do not form insolubility 3. - Soluble Insoluble NH4Br CoBr2 Pb NO32 PbCl2 BaSO4 CuCO3 Note.
You are provided with solid lead II nitrate Pb NO32 and solid sodium iodide NaI. The molar mass of PbNO32 is 3312 g. What is the molar mass of PbNO32.
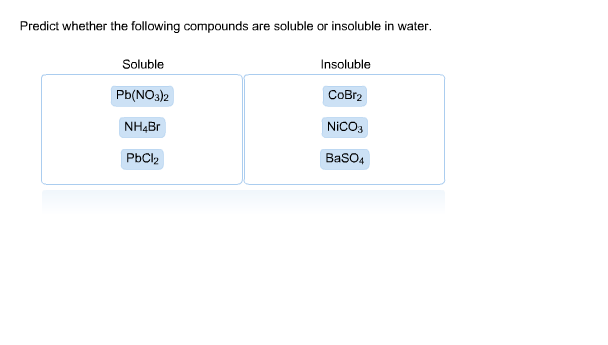
Solved Predict Whether The Following Compounds Are Soluble Chegg Com


Comments
Post a Comment